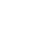CAS: 77472-71-0
Contact me
Email : salesexecutive1@yeah.net
whatsapp: +8618931626169
wickr: lilywang
Consuetudinem
N-formyl derivativum 4 adeptus est refluxus 2 in acido formico per 5 horas in magno cede.Acidum aceticum N'-[2-(7-hydroxy-2-oxo-2 H-chromen-4-yl)-acetyl]-hydrazidum (5) ex refluxu acetylationis 2 in acido acetico bono cede consecutum est.Compositum 6 obtinetur etiam refluxus 2 cum 3-(2-bromoacetylo) -4-hydroxy-2-h-chromeno-2-unum in ethanol.Compone 2 reagit cum phenyl isothiocyanate in ethanolo ad cella temperiem obtinendam 4-phenyl-1-(7-hydroxy-2-oxo-2 H-chromeno-4-acetyl-)-thiosemicarbazide (7).
3-{2-[2-(7-hydroxy-2-oxo-2 H-chromen-4-yl)-acetyl]hydrazono}butanoate (8) ethyl niensis (8), 3-{2-[2-( 7-hydroxy-2-oxo-2 H-Chromen-4-yl)-acetyl] Hydrazono} Butanoate (8), Proventus 48% (Option 2).Structura compositorum 4-8 data eorum analyticis eorumque IR et 1 H-NMR spectris determinata est.Effusio infrared ob NH, OH et C=O functiones ad 3450-3000 et 1728-1610cm-1 respective apparet.Vincula effusio sociata coram aliis coetibus functionis adsunt in regione expectata.Imaginis compositi 1 H-NMR unum apicem ostendit in 8.03-10.58 ppm regione protons NH et OH respondentem.Compone 4-8